NICE says yes to Novartis’ multiple sclerosis therapy Kesimpta
pharmaphorum
APRIL 20, 2021
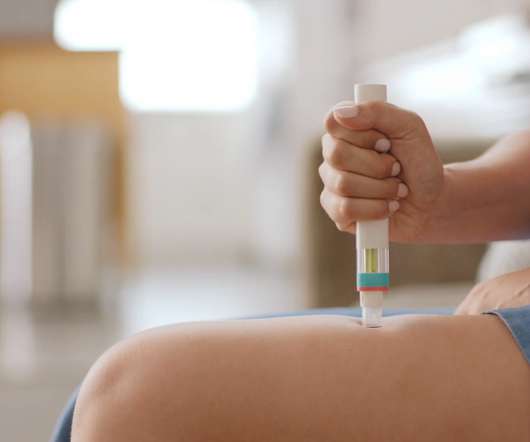
The cost-effectiveness agency has said that anti-CD20 antibody Kesimpta (ofatumumab) can be prescribed via the NHS in England and Wales as a treatment for adults with RMS with active disease, as either a first-line therapy or after alternative drugs have been tried. billion by 2028. billion in sales in 2028.











Let's personalize your content